A Century of Vitamin E: Early Milestones and Future Directions in Animal Nutrition
Abstract
:1. Introduction
2. Early Discoveries and Understanding of Vitamin E
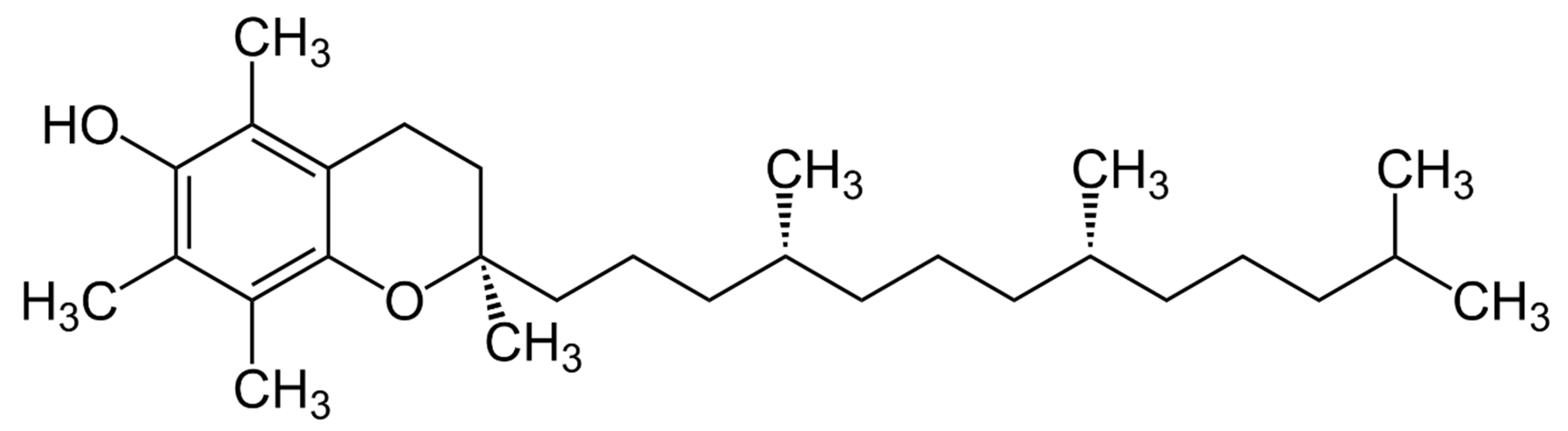
3. Vitamin E’s Chemical Structure and Biological Activity
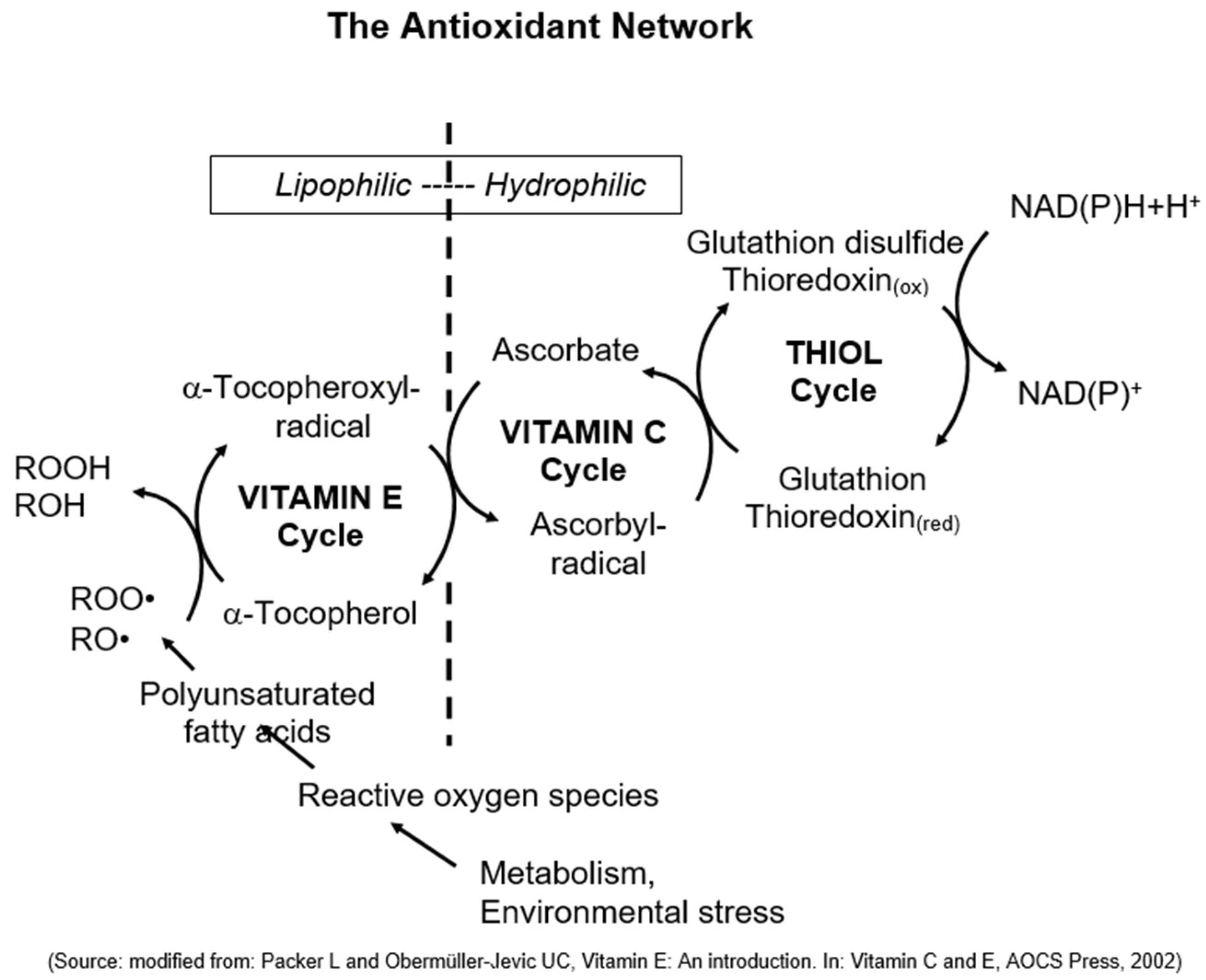
4. The Discovery of Vitamin E’s Unique Physiological Function as Chain-Breaking Antioxidant and the Antioxidant Network
5. The Synergy of Vitamin E, Vitamin C, and Selenium
6. The Evolution of Vitamin E Production: From Natural Sources to Synthetic Pathways and Standardized Potency Units
7. Early Experiments on Vitamin E and Its Effects on Animal Health
8. Current Status and Future Research of Vitamin E in Animal Nutrition
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Doğru Pekiner, B. Vitamin E as an antioxidant. J. Fac. Pharm. Ankara 2003, 32, 243–267. [Google Scholar] [CrossRef] [Green Version]
- Burton, G.W.; Joyce, A.; Ingold, K.U. First proof that vitamin E is major lipid-soluble, chain-breaking antioxidant in human blood plasma. Lancet 1982, 2, 327. [Google Scholar] [CrossRef] [PubMed]
- Traber, M.G.; Atkinson, J. Vitamin E, antioxidant and nothing more. Free Radic. Biol. Med. 2007, 43, 4–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Idamokoro, E.M.; Falowo, A.B.; Oyeagu, C.E.; Afolayan, A.J. Multifunctional activity of vitamin E in animal and animal products: A review. Anim. Sci. J. 2020, 91, e13352. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Khan, M.Z.; Ma, Y.; Alugongo, G.M.; Ma, J.; Chen, T.; Khan, A.; Cao, Z. The Antioxidant Properties of Selenium and Vitamin E Their Role in Periparturient Dairy Cattle Health Regulation. Antioxidants 2021, 10, 1555. [Google Scholar] [CrossRef] [PubMed]
- Hogan, J.S.; Weiss, W.P.; Smith, K.L. Role of Vitamin E and Selenium in Host Defense Against Mastitis. J. Dairy Sci. 1993, 76, 2795–2803. [Google Scholar] [CrossRef]
- Cusack, P.; McMeniman, N.; Rabiee, A.; Lean, I. Assessment of the effects of supplementation with vitamin E on health and production of feedlot cattle using meta-analysis. Prev. Vet. Med. 2009, 88, 229–246. [Google Scholar] [CrossRef]
- Chandra, G.; Aggarwal, A.; Singh, A.K.; Kumar, M.; Upadhyay, R.C. Effect of vitamin E and zinc supplementation on energy metabolites, lipid peroxidation, and milk production in peripartum sahiwal cows. Asian-Australas. J. Anim. Sci. 2013, 26, 1569–1576. [Google Scholar] [CrossRef] [Green Version]
- Rengaraj, D.; Hong, Y.H. Effects of dietary vitamin E on fertility functions in poultry species. Int. J. Mol. Sci. 2015, 16, 9910–9921. [Google Scholar] [CrossRef] [Green Version]
- Surai, P.F.; Fisinin, V.I.; Karadas, F. Antioxidant systems in chick embryo development. Part 1. Vitamin E, carotenoids and selenium. Anim. Nutr. 2016, 2, 1–11. [Google Scholar] [CrossRef]
- Cheah, K.S.; Cheah, A.M.; Krausgrill, D.I. Effect of dietary supplementation of vitamin E on pig meat quality. Meat Sci. 1995, 39, 255–264. [Google Scholar] [CrossRef]
- Corino, C.; Oriani, G.; Pantaleo, L.; Pastorelli, G.; Salvatori, G. Influence of dietary vitamin E supplementation on “heavy” pig carcass characteristics, meat quality, and vitamin E status. J. Anim. Sci. 1999, 77, 1755–1761. [Google Scholar] [CrossRef]
- Lu, T.; Harper, A.F.; Zhao, J.; Estienne, M.J.; Dalloul, R.A. Supplementing antioxidants to pigs fed diets high in oxidants: I. Effects on growth performance, liver function, and oxidative status. J. Anim. Sci. 2014, 92, 5455–5463. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Dal Jang, Y.; Rentfrow, G.K.; Azain, M.J.; Lindemann, M.D. Effects of dietary vitamin E and fat supplementation in growing-finishing swine fed to a heavy slaughter weight of 150 kg: I. Growth performance, lean growth, organ size, carcass characteristics, primal cuts, and pork quality. J. Anim. Sci. 2022, 100, skac081. [Google Scholar] [CrossRef]
- McDowell, L.R. Vitamin nutrition of livestock animals: Overview from vitamin discovery to today. Can. J. Anim. Sci. 2006, 86, 171–179. [Google Scholar] [CrossRef]
- Evans, H.M.; Bishop, K.S. On the existence of a hitherto unrecognized dietary factor essential for reproduction. Science 1922, 56, 650–665. [Google Scholar] [CrossRef] [Green Version]
- Sure, B. Dietary requirements for reproduction. II. The existence of a specific vitamin for reproduction. J. Biol. Chem. 1924, 58, 693–703. [Google Scholar] [CrossRef]
- Evans, H.M. Invariable occurrence of male sterility with dietaries lacking fat soluble vitamine E. Proc. Natl. Acad. Sci. USA 1925, 11, 373–377. [Google Scholar] [CrossRef]
- Evans, H.M.; Burr, G.O. The anti-sterility fat soluble vitamin E. Proc. Natl. Acad. Sci. USA 1925, 11, 334–341. [Google Scholar] [CrossRef]
- Evans, H.M.; Emerson, O.H.; Emerson, G.A. The isolation from wheat germ oil of an alcohol, α-tocopherol, having the properties of vitamin E. J. Biol. Chem. 1936, 113, 319–332. [Google Scholar] [CrossRef]
- Emerson, O.H.; Emersox, G.A.; Mohammad, A.; Evans, H.M. The chemistry of vitamin E-tocopherols from various sources. J. Biol. Chem. 1937, 122, 99–107. [Google Scholar] [CrossRef]
- Stern, M.H.; Robeson, C.D.; Weisler, L.; Baxter, J.G. δ-Tocopherol. I. Isolation from Soybean Oil and Properties. J. Am. Chem. Soc. 1947, 69, 869–874. [Google Scholar] [CrossRef] [PubMed]
- Fernholz, E. On the constitution of α-tocopherol. J. Am. Chem. Soc. 1938, 60, 700–705. [Google Scholar] [CrossRef]
- Fernholz, E. The thermal decomposition of alpha-tocopherol. J. Am. Chem. Soc. 1937, 59, 1154–1155. [Google Scholar] [CrossRef]
- Karrer, P.; Fritzsehe, H.; Ringier, B.H.; Salomon, H. α-Tocopherol. Helv. Chim. Acta 1938, 21, 520–525. [Google Scholar] [CrossRef]
- Karrer, P. Vitamin E und verwandte Verbindungen. Helv. Chim. Acta 1939, 22, 334–350. [Google Scholar] [CrossRef]
- Bergel, F.; Jacob, A.; Todd, A.R.; Work, T.S. Vitamin E Synthesis of α-Tocopherol. Nature 1938, 142, 36. [Google Scholar] [CrossRef]
- Smith, L.I.; Ungnade, H.E.; Prichard, W.W. The Chemistry of Vitamin E. I. The Structure and Synthesis of α-Tocopherol. Science 1938, 88, 37–38. [Google Scholar] [CrossRef]
- Isler, O. Die Stabilisierung von d,l-α-Tocopherol. Helv. Chim. Acta 1938, 21, 1756–1759. [Google Scholar] [CrossRef]
- John, W. Über das Cumo-tokopherol, einen neuen Faktor der Vitamin E-Gruppe. Z. Physiol. Chem. 1937, 250, 11–24. [Google Scholar] [CrossRef]
- John, W. Zum Beweis der Chromanstruktur des α-Tokopherols. Naturwissenschaften 1938, 5, 21–22. [Google Scholar] [CrossRef]
- Mattill, H.A.; Carman, J.S.; Clayton, M.M. The nutritive properties of milk. III. The effectiveness of the X substance in preventing sterility in rats on milk rations high in fat. J. Biol. Chem. 1924, 61, 729–740. [Google Scholar] [CrossRef]
- Mattill, H.A. The oxidative destruction of vitamins A and E and the protective action of certain vegetable oils. J. Am. Med. Assoc. 1927, 89, 1505–1508. [Google Scholar] [CrossRef]
- Cummings, M.J.; Mattill, H.A. The auto-oxidation of fats with reference to their destructive effect on vitamin E. J. Nutr. 1931, 3, 421–432. [Google Scholar] [CrossRef]
- Tappel, A.L.; Zalkin, H. Inhibition of lipide peroxidation in mitochondria by vitamin E. Arch. Biochem. Biophys. 1959, 80, 333–336. [Google Scholar] [CrossRef]
- Tappel, A.L.; Zalkin, H. Inhibition of lipid peroxidation in microsomes by vitamin E. Nature 1960, 4705, 35. [Google Scholar] [CrossRef]
- Burton, G.W.; Ingold, K.U. Autoxidation of biological molecules. 1. The antioxidant activity of vitamin E and related chain-breaking phenolic antioxidants in vitro. J. Am. Chem. Soc. 1981, 103, 6472–6477. [Google Scholar] [CrossRef]
- Burton, G.W.; Ingold, K.U. Vitamin E as an in vitro and in vivo antioxidant. Ann. N. Y. Acad. Sci. 1989, 570, 7–22. [Google Scholar] [CrossRef]
- Packer, L.; Smith, J.R. Extension of the lifespan of cultured normal human diploid cells by vitamin E: A reevaluation. Proc. Natl. Acad. Sci. USA 1977, 74, 1640–1641. [Google Scholar] [CrossRef]
- Packer, L.; Landvik, S. Vitamin E: Introduction to biochemistry and health benefits. Ann. N. Y. Acad. Sci. 1989, 570, 1–6. [Google Scholar] [CrossRef]
- Packer, L.; Obermüller-Jevic, U.C. Vitamin E: An introduction. In The Antioxidant Vitamins C and E; Packer, L., Traber, M.G., Kramer, K., Eds.; AOCS Press: Champaign, IL, USA, 2002; pp. 133–151. [Google Scholar]
- Hondal, R.J. Selenium vitaminology: The connection between selenium, vitamin C, vitamin E, and ergothioneine. Curr. Opin. Chem. Biol. 2023, 75, 102328. [Google Scholar] [CrossRef] [PubMed]
- Diplock, A.T. The role of vitamin E in biological membranes. Ciba Found. Symp. 1983, 101, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.; Omage, S.O.; Börmel, L.; Kluge, S.; Schubert, M.; Wallert, M.; Lorkowski, S. Vitamin E and Metabolic Health: Relevance of Interactions with Other Micronutrients. Antioxidants 2022, 11, 1785. [Google Scholar] [CrossRef] [PubMed]
- Przybyło, M.; Lagner, M. On the physiological and cellular homeostais of ascorbate. Cell. Mol. Biol. Lett. 2020, 25, 32. [Google Scholar] [CrossRef]
- Kaźmierczak-Barańska, J.; Boguszewska, K.; Adamus-Grabicka, A.; Karwowski, B.T. Two Faces of Vitamin C-Antioxidative and Pro-Oxidative Agent. Nutrients 2020, 12, 1501. [Google Scholar] [CrossRef]
- Traber, M.G.; Stevens, J.F. Vitamins C and E: Beneficial effects from a mechanistic perspective. Free Radic. Biol. Med. 2011, 51, 1000–1013. [Google Scholar] [CrossRef] [Green Version]
- Lubos, E.; Loscalzo, J.; Handy, D.E. Glutathione peroxidase-1 in health and disease: From molecular mechanisms to therapeutic opportunities. Antioxid. Redox. Signal. 2011, 15, 1957–1997. [Google Scholar] [CrossRef] [Green Version]
- Kang, D.; Lee, J.; Wu, C.; Guo, X.; Lee, B.J.; Chun, J.S.; Kim, J.H. The role of selenium metabolism and selenoproteins in cartilage homeostasis and arthropathies. Exp. Mol. Med. 2020, 52, 1198–1208. [Google Scholar] [CrossRef]
- Desai, I.D.; Scott, M.L. Mode of action of selenium in relation to biological activity of tocopherols. Arch. Biochem. Biophys. 1965, 110, 309–315. [Google Scholar] [CrossRef]
- Hafeman, D.G.; Hoekstra, W.G. Lipid peroxidation in vivo during vitamin E and selenium deficiency in the rat as monitored by ethane evolution. J. Nutr. 1977, 107, 666–672. [Google Scholar] [CrossRef]
- Walsh, D.M.; Kennedy, S.; Blanchflower, W.J.; Goodall, E.A.; Kennedy, D.G. Vitamin E and selenium deficiencies increase indices of lipid peroxidation in muscle tissue of ruminant calves. Int. J. Vitam. Nutr. Res. 1993, 63, 188–194. [Google Scholar]
- Sokmen, B.; Basaraner, H.; Yanardag, R. Combined effects of treatment with vitamin C, vitamin E and selenium on the skin of diabetic rats. Hum. Exp. Toxicol. 2013, 32, 379–384. [Google Scholar] [CrossRef]
- Akbari, A.; Jelodar, G.; Nazif, S.; Sajedianfard, J. An overview of the characteristics and function of vitamin C in Various tissues: Relying on its antioxidant function. Zahedan J. Res. Med. Sci. 2016, 18, 4037. [Google Scholar] [CrossRef] [Green Version]
- Bendich, A. Vitamin E and immune functions. Basic Life Sci. 1988, 49, 615–620. [Google Scholar] [CrossRef]
- Kiani, A.K.; Dhuli, K.; Donato, K.; Aquilanti, B.; Velluti, V.; Matera, G.; Iaconelli, A.; Connelly, S.T.; Bellinato, F.; Gisondi, P.; et al. Main nutritional deficiencies. J. Prev. Med. Hyg. 2022, 63, E93–E101. [Google Scholar] [CrossRef]
- Eggersdorfer, M.; Laudert, D.; Létinois, U.; McClymont, T.; Medlock, J.; Netscher, T.; Bonrath, W. One Hundred Years of Vitamins—A Success Story of the Natural Sciences. Angew. Chem. Int. Ed. 2012, 51, 12960–12990. [Google Scholar] [CrossRef]
- Anonymous. Vitamin E. Nature 1940, 145, 345. [Google Scholar] [CrossRef] [Green Version]
- Grandel, F. Das Vitamin E, seine Bedeutung bei Mensch, Tier und Pflanze. Angew. Chem. 1939, 24, 420–426. [Google Scholar] [CrossRef]
- Hume, E.M. Standardization of vitamin E. Nature 1941, 148, 472–473. [Google Scholar] [CrossRef] [Green Version]
- Brubacher, G.; Wiss, O. Tocopherols: Standardization of Activity. In The Vitamins; Sebrell, H., Harris, R.S., Eds.; Academic Press: Cambridge, MA, USA, 1972; pp. 248–251. [Google Scholar] [CrossRef]
- Kappus, A.; Diplock, H.T. Tolerance and safety of Vitamin E: A toxicological position report. Free Radic. Biol. Med. 1992, 13, 55–74. [Google Scholar] [CrossRef]
- AWT (Arbeitsgemeinschaft für Wirkstoffe in der Tierernährung e.V.). Vitamins in Animal Nutrition: Vitamin E.; AgriMedia: Eisenberg, Germany, 2002; p. 15. ISBN 3-86037-167-3. [Google Scholar]
- Anonymous. ANRC acts on vitamin E, K standards. Feedstuffs 1962, 34, 34. [Google Scholar]
- Leeson, S.; Summers, J.D. Commercial Poultry Nutrition. In Scott’s Nutrition of the Chicken; Leeson, S., Ed.; Nottingham University Press: Nottingham, UK, 2005; p. 398. [Google Scholar]
- Kleyn, R.; Chrystal, P. Vitamins. In Broiler Nutrition: Masterclass; Context Products Ltd.: Leicestershire, UK, 2020; pp. 129–142. [Google Scholar]
- Haga, S.; Ishizaki, H.; Roh, S. The Physiological Roles of Vitamin E and Hypovitaminosis E in the Transition Period of High-Yielding Dairy Cows. Animals 2021, 11, 1088. [Google Scholar] [CrossRef] [PubMed]
- Van Vleet, J.F.; Ferrans, V.J. Etiologic factors and pathologic alterations in selenium-vitamin E deficiency and excess in animals and humans. Biol. Trace. Elem. Res. 1992, 33, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Darroch, C.S. Selenium and Vitamin E in Swine Nutrition. In Swine Nutrition; Lewis, A.J., Southern, L.L., Eds.; CRC Press: New York, NY, USA, 2000; pp. 281–314. [Google Scholar]
- Nielsen, M.M.; Hansen, A. Stability of vitamin E in wheat flour and whole wheat flour during storage. Cereal Chem. 2008, 85, 716–720. [Google Scholar] [CrossRef] [Green Version]
- Tan, Y.; Zhou, H.; Zhang, Z.; McClements, D.J. Bioaccessibility of oil-soluble vitamins (A, D, E) in plant-based emulsions: Impact of oil droplet size. Food Funct. 2021, 12, 3883–3897. [Google Scholar] [CrossRef]
- NASEM (National Academies of Sciences, Engineering, and Medicine). Nutrient Requirements of Poultry, 9th ed.; National Academy Press: Washington, DC, USA, 1994. [Google Scholar]
- Chen, Y.F.; Huang, C.F.; Liu, L.; Lai, C.H.; Wang, F.L. Concentration of vitamins in the 13 feed ingredients commonly used in pig diets. Anim. Feed Sci. Technol. 2019, 247, 1–8. [Google Scholar] [CrossRef]
- Choo, Y.K.; Kwon, H.J.; Oh, S.T.; Kang, C.W.; Kim, H.K.; Hong, E.C.; Heo, K.N.; Lee, S.K.; An, B.K. Growth performance and carcass characteristics of Korean native ducks fed diets with varying levels of limiting amino acids. Asian-Aust. J. Anim. Sci. 2014, 27, 518–523. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.H.; Qi, G.E.; Sim, J.S. Metabolizable energy and amino acid availability of full-fat seeds, meals, and oils of flax and canola. Poult. Sci. 1995, 74, 1341–1348. [Google Scholar] [CrossRef]
- Beeckman, A.; Vicca, J.; Van Ranst, G.; Janssens, G.P.J.; Fievez, V. Monitoring of vitamin E status of dry, early and mid-late lactating organic dairy cows fed conserved roughages during the indoor period and factors influencing forage vitamin E levels. J. Anim. Physiol. Anim. Nutr. 2010, 94, 736–746. [Google Scholar] [CrossRef]
- Mogensen, L.; Kristensen, T.; Søegaard, K.; Jensen, S.K.; Sehested, J. Alfa-tocopherol and beta-carotene in roughages and milk in organic dairy herds. Livest. Sci. 2012, 145, 44–54. [Google Scholar] [CrossRef]
- Lindqvist, H.; Nadeau, E.; Persson Waller, K.; Jensen, S.K.; Johansson, B. Effects of RRR-α-tocopheryl acetate supplementation during the transition period on vitamin status in blood and milk of organic dairy cows during lactation. Livest. Sci. 2011, 142, 155–163. [Google Scholar] [CrossRef]
- Johansson, B.; Persson Waller, K.; Jensen, S.K.; Lindqvist, H.; Nadeau, E. Status of vitamins E and a and β-carotene and health in organic dairy cows fed a diet without synthetic vitamins. J. Dairy Sci. 2014, 97, 1682–1692. [Google Scholar] [CrossRef] [Green Version]
- Höjer, A.; Adler, S.; Martinsson, K.; Jensen, S.K.; Steinhsamn, H.; Thuen, T.; Gustavsson, A.-M. Effect of legume–grass silages and a-tocopherol supplementation on fatty acid composition and a-tocopherol, b-carotene and retinol concentrations in organically produced bovine milk. Livest. Sci. 2012, 148, 268–281. [Google Scholar] [CrossRef]
- Kidane, A.; Nesheim, I.L.; Larsen, H.J.S.; Thuen, E.; Jensen, S.K.; Steinshamn, H. Effects of supplementing mid-lactation dairy cows with seaweed and vitamin E on plasma and milk α -tocopherol and antibody response to immunization. J. Agric. Sci. 2015, 153, 929–942. [Google Scholar] [CrossRef] [Green Version]
- Goettsch, M.; Pappenheimer, A.M. Nutritional muscular dystrophy in the guinea pig and rabbit. J. Exp. Med. 1931, 54, 145–165. [Google Scholar] [CrossRef] [Green Version]
- Pappenheimer, A.M.; Goettsch, M. A cerebellar disorder in chicks, apparently of nutritional origin. J. Exp. Med. 1931, 53, 11–26. [Google Scholar] [CrossRef]
- Goettsch, M.; Pappenheimer, A.M. Nutritional myopathy in ducklings. J. Exp. Med. 1934, 59, 35–42. [Google Scholar] [CrossRef] [Green Version]
- Dam, H.; Glavind, J. Alimentary Exudative Diathesis. Nature 1938, 142, 1077–1078. [Google Scholar] [CrossRef]
- Machlin, L.J.; Gordon, R.S. Etiology of Exudative Diathesis, Encephalomalacia, and Muscular Degeneration in the Chicken. Poult. Sci. 1961, 41, 473–477. [Google Scholar] [CrossRef]
- Harris, P.L.; Ludwig, M.I. Relative vitamin E potency of natural and of synthetic α-tocopherol. J. Biol. Chem. 1949, 179, 111–115. [Google Scholar] [CrossRef]
- Bunyan, J.; McHale, D.; Green, J.; Marcinkiewicz, S. Biological potencies of epsilon- and zeta-1-tocopherol and 5-methyltocol. Br. J. Nutr. 1961, 15, 253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marsh, J.A.; Dietert, R.R.; Combs, G.F. Influence of dietary selenium and vitamin E on the humoral immune response of chicks. Proc. Soc. Exp. Biol. Med. 1981, 166, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Peplowski, M.A.; Mahan, D.C.; Murray, F.A.; Moxon, A.L.; Cantor, A.H.; Ekstrom, K.E. Effect of dietary and injectable vitamin E and selenium in weanling swine antigenically challenged with sheep red blood cells. J. Anim. Sci. 1981, 51, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Blaxter, K.L. The significance of selenium and vitamin E in nutrition. Muscular dystrophy in farm animals: Its cause and prevention. Proc. Nutr. Soc. 1962, 21, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Vos, J.; Hulstaert, C.E.; Molenaar, I. Nutritional myopathy in ducklings: A growth rate-dependent symptom of “tissue peroxidosis” due to a net nutritional shortage of vitamin E plus selenium in skeletal muscle. Ann. Nutr. Metab. 1981, 25, 299–306. [Google Scholar] [CrossRef]
- Menzies, P.; Langs, L.; Boermans, H.; Martin, J.; McNally, J. Myopathy and hepatic lipidosis in weaned lambs due to vitamin E deficiency. Can. Vet. J. 2004, 45, 244–247. [Google Scholar]
- Olson, R.E. Vitamin E and its relation to heart disease. Circulation 1973, 48, 179–184. [Google Scholar] [CrossRef] [Green Version]
- Scott, M.L. Advances in our understanding of vitamin E. Fed. Proc. Fed. Am. Soc. Exp. Biol. 1980, 39, 2736–2739. [Google Scholar]
- Gitler, C.; Sunde, M.L.; Baumann, C.A. Effect of certain necrosis-preventing factors on hemolysis in vitamin E-deficient rats and chicks. J. Nutr. 1958, 65, 397–407. [Google Scholar] [CrossRef]
- Fryer, M.J. Vitamin E as a protective antioxidant in progressive renal failure. Nephrology 2000, 5, 1–7. [Google Scholar] [CrossRef]
- Nafstad, I.; Tollersrud, S. The vitamin E-deficiency syndrome in pigs. I. Pathological changes. Acta Vet. Scand. 1970, 11, 452–480. [Google Scholar] [CrossRef]
- Hackett, M. Vitamins. In Chemical Economics Handbook; IHS Markit: London, UK, 2021; pp. 38–39. [Google Scholar]
- Chen, C.; Wang, Z.; Li, J.; Li, Y.; Huang, P.; Ding, X.; Yin, J.; He, S.; Yang, H.; Yin, Y. Dietary vitamin E affects small intestinal histomorphology, digestive enzyme activity, and the expression of nutrient transporters by inhibiting proliferation of intestinal epithelial cells within jejunum in weaned piglets. J. Anim. Sci. 2019, 97, 1212–1221. [Google Scholar] [CrossRef]
- Choi, Y.; Lee, S.; Kim, S.; Lee, J.; Ha, J.; Oh, H.; Lee, Y.; Kim, Y.; Yoon, Y. Vitamin E (α-tocopherol) consumption influences gut microbiota composition. Int. J. Food. Sci. 2020, 71, 221–225. [Google Scholar] [CrossRef]
- Calik, A.; Emami, N.K.; White, M.B.; Walsh, M.C.; Romero, L.F.; Dalloul, R.A. Influence of dietary vitamin E and selenium supplementation on broilers subjected to heat stress, Part I: Growth performance, body composition and intestinal nutrient transporters. Poult. Sci. 2022, 101, 101857. [Google Scholar] [CrossRef]
- Khalifa, O.A.; Al Wakeel, R.A.; Hemeda, S.A.; Abdel-Daim, M.M.; Albadrani, G.M.; El Askary, A.; Fadl, S.E.; Elgendey, F. The impact of vitamin E and/or selenium dietary supplementation on growth parameters and expression levels of the growth-related genes in broilers. BMC Vet. Res. 2021, 17, 251. [Google Scholar] [CrossRef]
- Rehman, Z.U.; Meng, C.; Sun, Y.; Mahrose, K.M.; Umar, S.; Ding, C.; Munir, M. Pathobiology of Avian avulavirus 1: Special focus on waterfowl. Vet. Res. 2018, 49, 94. [Google Scholar] [CrossRef] [Green Version]
- Jian, L.; Xue, Y.; Gao, Y.; Wang, B.; Qu, Y.; Li, S.; Li, H.; Li, Z.; Wang, B.; Luo, H. Vitamin E Can Ameliorate Oxidative Damage of Ovine Hepatocytes In Vitro by Regulating Genes Expression Associated with Apoptosis and Pyroptosis, but Not Ferroptosis. Molecules 2021, 26, 4520. [Google Scholar] [CrossRef]
- Belanche, A.; Kingston-Smith, A.H.; Newbold, C.J. An integrated multi-omics approach reveals the effects of supplementing grass or grass hay with vitamin E on the rumen microbiome and its function. Front. Microbiol. 2016, 7, 905. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.; Guo, Y.; Zhang, J.; Deng, M.; Xian, Z.; Xiong, H.; Liu, D.; Sun, B. High-Dose Vitamin E Supplementation Can Alleviate the Negative Effect of Subacute Ruminal Acidosis in Dairy Cows. Animals 2023, 13, 486. [Google Scholar] [CrossRef]
- Juárez, M.; Dugan, M.E.; Aalhus, J.L.; Aldai, N.; Basarab, J.A.; Baron, V.S.; McAllister, T.A. Effects of vitamin E and flaxseed on rumen-derived fatty acid intermediates in beef intramuscular fat. Meat Sci. 2011, 88, 434–440. [Google Scholar] [CrossRef]
- Lee, G.Y.; Han, S.N. The Role of Vitamin E in Immunity. Nutrients 2018, 10, 1614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hartmann, M.S.; Mousavi, S.; Bereswill, S.; Heimesaat, M.M. Vitamin E as promising adjunct treatment option in the combat of infectious diseases caused by bacterial including multi-drug resistant pathogens—Results from a comprehensive literature survey. Eur. J. Microbiol. Immunol. 2020, 10, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Hosain, M.Z.; Kabir, S.M.L.; Kamal, M.M. Antimicrobial uses for livestock production in developing countries. Vet. World 2021, 14, 210–221. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xu, X.; Su, G.; Shi, B.; Shan, A. High concentration of vitamin E supplementation in sow diet during the last week of gestation and lactation affects the immunological variables and antioxidative parameters in piglets. J. Dairy Res. 2017, 84, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Gaykwad, C.K.; De, U.K.; Jadhav, S.E.; Chethan, G.E.; Akhilesh; Sahoo, N.R.; Mondal, D.B.; Gaur, G.K.; Verma, M.R.; Chaudhuri, P. Adding α-tocopherol-selenium and ascorbic acid to periparturient sow diets influences hemogram, lipid profile, leptin, oxidant/antioxidant imbalance, performance and neonatal piglet mortality. Res. Vet. Sci. 2019, 125, 360–369. [Google Scholar] [CrossRef]
- Mahmood, N.; Hameed, A.; Hussain, T. Vitamin E and Selenium Treatment Alleviates Saline Environment-Induced Oxidative Stress through Enhanced Antioxidants and Growth Performance in Suckling Kids of Beetal Goats. Oxid. Med. Cell. Longev. 2020, 2020, 4960507. [Google Scholar] [CrossRef]
- Zdanowska-Sąsiadek, Ż.; Michalczuk, M.; Damaziak, K.; Niemiec, J.; Poławska, E.; Gozdowski, D.; Różańska, E. Effect of vitamin E supplementation on growth performance and chicken meat quality. Eur. Poult. Sci. 2016, 80, 152. [Google Scholar] [CrossRef]
- Bellés, M.; del Mar Campo, M.; Roncalés, P.; Beltrán, J.A. Supranutritional doses of vitamin E to improve lamb meat quality. Meat Sci. 2019, 149, 14–23. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Wang, J.; Zhang, H.; Wu, S.; Yue, H.; Wan, X.; Yang, H.; Wang, Z.; Qi, G. Vitamin E Supplementation Enhances Lipid Oxidative Stability via Increasing Vitamin E Retention, Rather Than Gene Expression of MAPK-Nrf2 Signaling Pathway in Muscles of Broilers. Foods 2021, 10, 2555. [Google Scholar] [CrossRef]
- Trombetti, F.; Minardi, P.; Mordenti, A.L.; Badiani, A.; Ventrella, V.; Albonetti, S. The Evaluation of the Effects of Dietary Vitamin E or Selenium on Lipid Oxidation in Rabbit Hamburgers: Comparing TBARS and Hexanal SPME-GC Analyses. Foods 2022, 11, 1911. [Google Scholar] [CrossRef]
- Jose, C.G.; Jacob, R.H.; Pethick, D.W.; Gardner, G.E. Short term supplementation rates to optimise vitamin E concentration for retail colour stability of Australian lamb meat. Meat Sci. 2016, 111, 101–109. [Google Scholar] [CrossRef] [Green Version]
- Flachowsky, G. Vitamin E-transfer from feed into pig tissues. J. Appl. Anim. Res. 2000, 17, 69–80. [Google Scholar] [CrossRef] [Green Version]
- Berges, E. Importance of vitamin E in the oxidative stability of meat: Organoleptic qualities and consequences. In Feed Manufacturing in the Mediterranean Region: Recent Advances in Research and Technology; Brufau, J., Tacon, A., Eds.; CIHEAM: Zaragoza, Spain, 1999; pp. 347–363. [Google Scholar]
- Marusich, W.L.; De Ritter, E.; Ogrinz, E.F.; Keating, J.; Mitrovic, M.; Bunnell, R.H. Effect Supplemental Vitamin in Control of Rancidity in Poultry Meat; Animal Health Research Department and Product Development Department, Hoffmann-La Roche Inc.: Nutley, NJ, USA, 1994. [Google Scholar]
- Sünder, A.; Richter, G.; Flachowsky, G. EinfluB unterschiedlich hoher Vitamin E-Konzentrationen im Legehennenfutter auf den Vitamin E-Transfer ins Huhnerei. Proc. Soc. Nutr. Physiol. 1997, 6, 147. [Google Scholar]
- Yeargan, M.R.; Oshidari, S.; Mitchell, G.E., Jr.; Tucker, R.E.; Schelling, G.T.; Hemken, R.W. Mammary transfer of vitamin E in cows treated with vitamin A or linoleic acid. J. Dairy Sci. 1979, 62, 1734–1738. [Google Scholar] [CrossRef]

| Active Substance | Unit | Conversion Factors of Vitamin E Forms to Active Substance | |
|---|---|---|---|
| Tocopherol | mg | 1 mg dl-α-tocopheryl acetate | = 1 IU |
| Bioequivalence of various tocopherols | |||
| 1 mg d-α-tocopherol | = 1.49 IU | ||
| 1 mg dl-α-tocopherol | = 1.10 IU | ||
| 1 mg dl-α-tocopheryl acetate | = 1.00 IU | ||
| 1 mg dl-β-tocopherol | = 0.33 IU | ||
| 1 mg dl-δ-tocopherol | = 0.25 IU | ||
| 1 mg dl-γ-tocopherol | = 0.01 IU | ||
| Feedstuff | mg/kg | Reference |
|---|---|---|
| Corn | 18.7, 22.0 | [72,73] |
| Wheat | 7.3, 13.0 | [72,73] |
| Wheat bran | 19.8 | [73] |
| Soybean meal | 2.4, 2.5, 3.4 | [72,73,74] |
| Rapeseed meal | 8.7, 13.0 | [73,74,75] |
| Sunflower seed meal | 1 | [73] |
| Corn silage | 4.5, 13.0 | [76,77] |
| Hay | 4 | [76] |
| Fresh grass | 30, 36 | [78,79] |
| Grass clover silage | 39 | [80,81] |
| Form | Relative Efficiency |
|---|---|
| α-tocopherol | 100% |
| β-tocopherol | 15–40% |
| γ-tocopherol | 1–20% |
| δ-tocopherol | 1% |
| α-tocotrienol | 15–30% |
| β-tocotrienol | 1–5% |
| γ-tocotrienol | 1% |
| δ-tocotrienol | 1% |
| Disorder | Animal Model | Compromised Organ/Tissue | Reference |
|---|---|---|---|
| Immune deficiency | Chick, pig | Mononuclear phagocyte system | [89,90] |
| Myopathic disorders | Rabbit, duck, lamb, calf, turkey, chicken | Heart, skeletal muscles, gizzard | [91,92,93] |
| Reproductive dysfunction | |||
| embryonic apoptosis | Hen, turkey, cow | Embryonic circulatory system | [94] |
| infertility (male) | Rooster, rabbit | Testes | [95] |
| Kidney, pancreas, liver, brain, blood | |||
| necrobiosis | Pig | Liver | [94] |
| erythrocyte hemolysis | Chick, calf | Red blood cells | [96] |
| hypoproteinemia | Chick, turkey | Ricin | [94] |
| cerebral softening | Chick, duckling | Encephalon | [82] |
| hemorrhagic diathesis | Chick, turkey | Vascular system | [94] |
| nephrosis | Mink, rat | Renal tubular | [97] |
| yellow fat disease | Pig | Adipose tissue | [98] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shastak, Y.; Obermueller-Jevic, U.; Pelletier, W. A Century of Vitamin E: Early Milestones and Future Directions in Animal Nutrition. Agriculture 2023, 13, 1526. https://doi.org/10.3390/agriculture13081526
Shastak Y, Obermueller-Jevic U, Pelletier W. A Century of Vitamin E: Early Milestones and Future Directions in Animal Nutrition. Agriculture. 2023; 13(8):1526. https://doi.org/10.3390/agriculture13081526
Chicago/Turabian StyleShastak, Yauheni, Ute Obermueller-Jevic, and Wolf Pelletier. 2023. "A Century of Vitamin E: Early Milestones and Future Directions in Animal Nutrition" Agriculture 13, no. 8: 1526. https://doi.org/10.3390/agriculture13081526





